A New AI-Driven Approach to Identifying Critical Interaction Points in Cancer-Related Proteins
In the ongoing battle against cancer, one of the key frontiers lies at the molecular level: how proteins within our cells interact, signal, bind, and ultimately influence tumour growth or suppression. A recent breakthrough now offers a powerful new tool in this fight: an approach that uses artificial intelligence (AI) combined with molecular biophysics and network analysis to identify critical interaction points in cancer-related proteins. This article explores this development in depth—what it is, how it works, why it matters, and what it could mean for the future of cancer therapeutics.


Setting the stage: Why identifying interaction points matters
Proteins are the workhorses of biology. In cancer, many proteins involved in cell-division, signalling, DNA repair, immune regulation and other processes are mutated or dysregulated. These proteins rarely act alone—they often bind to other molecules (proteins, DNA, lipids) at specific surfaces or interfaces.
-
When a cancer-driver protein or its partner is mutated, the interaction interface may be altered, closing off a binding event or creating a false binding event.
-
Identifying where on the protein an interaction happens is crucial for targeting that interaction with a drug, antibody or engineered protein.
-
Traditional methods—X-ray crystallography, NMR, mutagenesis screens—are effective but slow, expensive and sometimes unable to capture dynamic or transient interactions.
So, the challenge: Can we more rapidly and precisely discover the critical binding sites / interaction hotspots on cancer-relevant proteins? The answer: increasingly yes—thanks to AI.
According to the National Cancer Institute (NCI), AI offers “unprecedented opportunity to advance our understanding of cancer” by detecting patterns in vast data that would otherwise elude human researchers. Cancer.gov
The recent breakthrough: AI meets biophysics and network-analysis
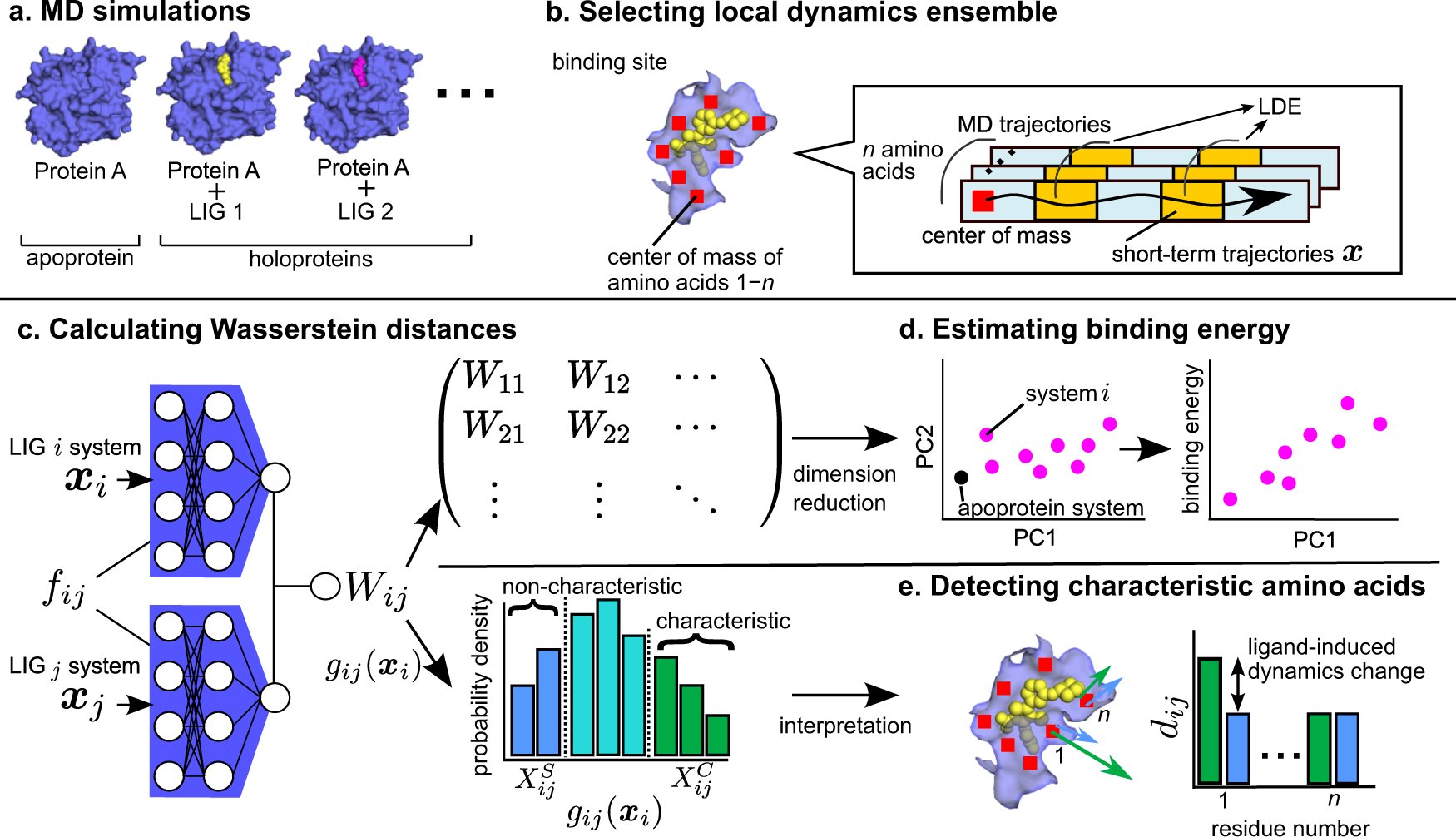
A landmark study by researchers at Auburn University (USA), in collaboration with University of Basel and ETH Zurich (Switzerland), introduced a novel integrative method that marries AI, molecular-dynamics simulation, and network-analysis to pinpoint binding sites on the immune-checkpoint protein PD‑L1 (programmed death-ligand 1). Phys.org+1
Key points of the approach:
-
Structure prediction via AI: They used tools based on AlphaFold2-type models to generate accurate 3D models of PD-L1 (and its complexes). Phys.org
-
Molecular dynamics (MD) simulations: The structures were then subjected to MD simulations to capture their dynamic behaviour—how the protein moves, flexes, and the interfaces that open or close. Phys.org
-
Dynamic network analysis: Treating the protein’s residues or atoms as nodes in a network, the team analysed how information (forces, interactions) flows through the structure under dynamic conditions to identify hotspots—nodes or edges critical to binding stability. Phys.org
-
Experimental validation: Predictions were validated using cross-linking mass spec, next-gen sequencing and other wet lab techniques to confirm that the predicted interaction sites truly matter biologically. Phys.org
The result: a set of previously under-recognized binding regions on PD-L1 which could inform next-generation immunotherapies.
Why this matters for cancer research & therapy
1. Precision targeting of immune checkpoint proteins
PD-L1 is an immune-checkpoint protein exploited by many cancers to evade immune attack. Drugs like Pembrolizumab (Keytruda) block PD-L1/PD-1 binding to release the immune brake. But better understanding of where and how PD-L1 binds its partners gives opportunities for improved design of therapeutic proteins, antibodies or small molecules. The new approach gives far more refined structural insight than was previously feasible. IndiaAI+1
2. Faster drug-target discovery
Traditional binding-site discovery is time-consuming. The AI-driven pipeline drastically speeds up identification of critical interaction points. Because the workflow integrates prediction, simulation and network analytics in silico before entering the lab, it cuts down wet-lab costs and timelines.
3. Broader applicability
Although the study focused on PD-L1, authors emphasise that their method is generalisable across other cancer-related proteins and even autoimmune or infectious disease protein interactions. Phys.org
4. Multi-omics integration and network perspective
Cancer is rarely a single protein issue—it involves networks of interacting proteins, changes in topology, signalling cascades and dysregulated hubs. This method adopts a network-analysis mindset, which aligns with emerging paradigms of systems biology in cancer. And the ability of AI to integrate multi-omics and structural data adds another powerful layer. MDPI+1
5. Towards personalised therapies
Because the method is computational, it could in future be adapted to patient-specific protein variants (mutants) to predict which interaction points in a given tumour’s proteome are most vulnerable. In other words: truly personalised structural therapeutics.
How the method works in more detail
Let’s walk through the workflow step-by-step, translating scientific jargon into accessible language.
Step 1: Data acquisition and structure modelling
-
The AI component uses tools like AlphaFold2 (or variants) to take amino acid sequence data of the protein (e.g., PD-L1) and generate a predicted 3D structure.
-
If a protein complex (protein + therapeutic partner) is modelled, the AI may predict how the two will dock.
-
Having a 3D model enables downstream simulation work.
Step 2: Molecular dynamics simulation
-
The predicted structure is placed in a simulation environment (water, ions, temperature conditions) and the atoms are allowed to move according to physical laws over time (nanoseconds to microseconds).
-
This captures how the protein/conformation changes, how binding pockets open/close, and reveals dynamics which static structures cannot show.
Step 3: Network representation & dynamic analysis
-
The protein is converted into a network graph: residues or atoms become nodes, interactions (distances, hydrogen bonds, van der Waals contacts) become edges.
-
As the simulation runs, the graph evolves: edges may form or break. Dynamic network metrics (e.g., betweenness centrality, edge-flux, network communities) highlight which nodes/edges are key conduits of structural communication.
-
These key nodes/edges are flagged as interaction hotspots because changes there disproportionately affect network integrity / binding stability.
Step 4: Ranking and prioritising interaction points
-
Hotspots are ranked by metrics: how frequently they participate in binding events during simulation; how central they are in the network; how accessible they are to drugs/antibodies.
-
AI classifiers may further refine this by combining structural, dynamic, and network features into a predictive model of likely druggability.
Step 5: Experimental validation
-
Wet-lab experiments (e.g., cross-linking mass spectrometry, mutagenesis, binding assays) test whether the predicted hotspots really do affect binding interactions or therapeutic efficacy.
-
Validated hotspots are then proposed as therapeutic targets.
Illustratively, the Auburn/ETH/Basel team applied this to PD-L1, successfully predicting binding-regions involved in its interaction with therapeutic proteins. Phys.org+1
Key findings from the study
Let’s summarise some of the study’s major findings:
-
The integrated AI–MD–network approach identified previously under-recognised binding regions on PD-L1 that could serve as epitopes for therapeutic proteins. Phys.org
-
The predictions were experimentally validated, boosting confidence in the computational pipeline (i.e., not purely speculative). Phys.org
-
The approach demonstrated how combining dynamic behaviour (rather than static structural snapshots) plus network-analysis gives deeper insight into binding interfaces.
-
The authors emphasised that this workflow can be scaled to many tumour-relevant proteins and their interactions, potentially accelerating the map of “druggable” interfaces across the cancer proteome. Phys.org
Challenges and considerations
While the approach is exciting, some cautionary notes and challenges remain.
Data quality and completeness
-
AI structure-prediction models are excellent but not perfect. Some proteins, especially large complexes, disordered regions or membrane proteins, remain challenging.
-
Molecular dynamics simulations require significant computational resources for long timescales and accurate force-fields.
-
Network models depend on accurate mapping of residue-interactions; simplifications may ignore water/solvent effects or rare conformational events.
Experimental validation remains essential
Computational prediction is compelling, but experimental confirmation remains the gold standard. As the study showed, validation (via cross-linking MS etc) is critical. Without it, predictions risk being “hypotheses on a screen”.
Druggability and accessibility
Even if an interaction point is identified, designing a molecule (small molecule, antibody, peptide) that accesses that hotspot, binds with sufficient affinity and has acceptable pharmacology is non-trivial. Also, some interfaces may be deeply buried or highly dynamic and thus less “druggable”.
Cancer heterogeneity and mutation context
Tumours are heterogeneous. A “critical interaction point” in one cancer subtype (or one mutant version of the protein) may not hold in another. The personalised-therapy promise requires adaptation of the pipeline to mutant sequences and varying cellular contexts.
Ethical, regulatory and translational hurdles
The path from hotspot identification to approved therapy remains long. Questions of safety, off-target effects, regulatory approval, cost and equitable access still apply.
Implications for the future of cancer therapeutics
Given the promising aspects and the remaining challenges, what might this approach mean for the future?
Accelerated target discovery
Large-scale implementation of this pipeline could yield a catalogue of actionable interaction hotspots across many cancer-relevant proteins. Researchers and pharmaceutical companies could then prioritise the most promising ones for drug development.
Personalized structural therapeutics
With tumour sequencing increasingly routine, one could imagine a workflow where a patient’s tumour-specific protein variants are modelled, hotspots predicted, and a bespoke therapeutic (e.g., antibody or engineered protein) is developed to engage that specific interface.
Combination therapies and network-informed targeting
Because the network-analysis emphasises “hub” residues or nodes critical to multiple interactions, therapies could be designed to disrupt network-connectivity rather than single interactions—offering more robust strategies to prevent resistance.
Integration with AI in other cancer research areas
This structural-interaction pipeline complements other AI-powered cancer research domains: imaging diagnostics, genomics, treatment-response prediction, biomarker discovery. As a holistic AI ecosystem in oncology matures, the synergy will grow. For example, the review “Integrating Omics Data and AI for Cancer Diagnosis and Prognosis” highlights how AI is already being used across genomics, proteomics, transcriptomics. MDPI
Cost & time efficiency
Traditional drug-discovery pipelines are slow and expensive. By front-loading binding-site discovery with computational workflows, the hope is to reduce the time from target-identification to lead molecule development—and thereby reduce cost. The study’s authors emphasised this potential. IndiaAI
SEO-friendly takeaways (for blog/website readers)
-
What has been developed? A novel AI-powered approach combining structure-prediction, molecular dynamics and network-analysis to identify critical binding/interaction sites in cancer-related proteins (e.g., PD-L1).
-
Why is this new? Previous methods often relied on static crystal structures or broad mutagenesis screens; this method integrates dynamic simulation + network modelling + AI for richer, faster insight.
-
Why does it matter for cancer? Because many cancer therapeutics target protein interactions (checkpoint proteins, signalling complexes); knowing where to bind improves design of drugs/antibodies.
-
What are the benefits? Faster target discovery, possibility of personalised therapeutics, broader applicability across cancer types, cost/time efficiency.
-
What are the caveats? High computational demands, need for accurate models and validation, translation to actual drug-molecule remains complex, tumour heterogeneity complicates generalisation.
-
What’s the future vision? A scalable catalogue of interaction hotspots across the cancer proteome; patient-specific structural modelling; network-informed combination therapies; integration into the broader AI-in-oncology ecosystem.
Frequently Asked Questions (FAQ)
Q1: What exactly is meant by “interaction point” in a protein?
An interaction point (or hotspot) refers to a region on a protein’s surface (or interface) where binding with another molecule (protein, antibody, ligand) is especially significant—either because many contacts form there, because altering that region disrupts binding, or because it is a “keystone” in the binding network.
Q2: How does AI help in this process of finding interaction points?
AI contributes at multiple levels:
-
Structure prediction (e.g., AlphaFold) to model proteins where no experimental structure exists.
-
Feature extraction from simulations or networks (residue centrality, binding-energy predictors).
-
Integration of heterogeneous data (sequence, dynamics, network metrics, mutation data) to prioritise hotspots.
Overall, AI helps identify patterns and relationships that would be difficult for humans or classical algorithms alone. MDPI+1
Q3: Why is molecular dynamics (MD) important here?
Static protein structures show one snapshot; MD allows you to see how the protein flexes, how pockets open/close, how interactions fluctuate over time. Many binding actions depend on dynamics—so incorporating MD makes the hotspot predictions more biologically realistic.
Q4: What is network‐analysis in the context of proteins?
Network-analysis treats components of the protein structure (residues or atoms) as nodes and their interactions (contacts, hydrogen bonds, energy flows) as edges. By analysing the network (central nodes, communication pathways, bottlenecks) you can find which parts of the protein structure are most important in maintaining functional connectivity – hence likely critical binding or regulatory sites.
Q5: Can this approach work for any cancer-related protein?
In principle yes—if the protein’s sequence is known, you can model its structure (or use existing models), run simulations, do network-analysis and AI integration. The Auburn/ETH/Basel team emphasize generalisability. Phys.org That said, practical issues (size, complexity, disorder, membrane environment) may make some proteins harder than others.
Q6: Does this guarantee a new drug or therapy will result?
No—this is a powerful tool for target discovery and interface identification. But moving from a hotspot to a successful drug involves many additional steps: molecule design, pharmacodynamics/pharmacokinetics, safety, clinical testing. This method accelerates the early stages of the pipeline but does not bypass the entire drug-development process.
Q7: How soon might this lead to patient-specific therapies?
While the vision is compelling (model a tumour-specific protein variant → predict hotspots → design bespoke binder), practical translation may take years. The computational tools are advancing rapidly, but scaling, regulatory pathways, manufacturing personalised biologics and cost-effectiveness need to be resolved.
Conclusion
The marriage of artificial intelligence, molecular-dynamics simulation and network analysis marks a significant step forward in our ability to identify critical interaction points in cancer-related proteins. By applying this integrative workflow to the checkpoint protein PD-L1, researchers have demonstrated how binding hotspots can be predicted with increasing accuracy and validated in the lab.
This approach holds promise not only for accelerating target discovery and therapeutic development, but eventually for realising structurally informed, personalised oncology treatments. It also underscores a broader trend: that in cancer research the real gains increasingly come not just from sequencing genomes or imaging tumours, but from understanding the molecular interactions at atomic resolution—and deploying AI to make sense of them.
Of course, challenges remain: ensuring accuracy, scalability, translation to therapies, dealing with tumour variability and moving into the clinic. But the pathway is clearer and faster than ever before. For researchers, clinicians and patients, that is very welcome progress.
For quick updates, follow our whatsapp –https://whatsapp.com/channel/0029VbAabEC11ulGy0ZwRi3j
https://bitsofall.com/https-yourblogdomain-com-ai-data-center-growth-in-the-us/
How to Build Your Own Database: A Step-by-Step Guide for Beginners
QeRL: NVFP4-Quantized Reinforcement Learning — Redefining Efficiency in AI Training and Inference